Abstract
Introduction:
Although 44 to 90% of CLL cases have kidney infiltration on autopsy, renal involvement generally goes undetected and renal failure is unusual (Da'As N et al, Eur J Haematol 2001). There is no standard therapy for nephrotic syndrome (NS) in CLL. Treatment of CLL itself may result in regression of NS in most patients and treatment with alkylating agents, cyclosporine A, fludarabine and even splenectomy have been shown to be effective. Controversy exists regarding effectiveness of rituximab in CLL-associated nephrotic syndrome. (Yahata et al, Am J Nephrol 2000). Here we report the first case, to our knowledge, of MBL/CLL-associated NS successfully treated with obinutuzumab.
Case Report:
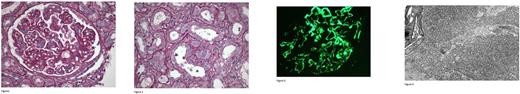
71 years old female presented to ER with hypertensive urgency and anasarca. Past medical history was relevant for progressive hypertension (HTN) for 1 year prior. Creatinine (Cr) was 1.9mg/dL, BUN 45mg/dL, urine protein was >300mg/dl on urinalysis and subsequent 24hr urine protein was 9824mg/24hrs. Patient (pt) had renal ultrasound revealing morphologically normal kidneys without evidence of renal artery stenosis. Echocardiogram was normal. CBC revealed anemia (Hgb 9.2mg/dl) and mild thrombocytopenia (plt 121), WBC count was 7.6 x 10e3/mcl with relative lymphocytosis (47.46 %, ALC 3.594 x10e3 /mcl). On left kidney core biopsy 25 glomeruli were seen by light microscopy, 1 of which was globally sclerotic. The remaining glomeruli showed diffuse duplication of basement membranes with cellular interposition and mild to moderate intracapillary hypercellularity with endothelial cell swelling and/or leukocyte accumulation (Figure 1). Proximal tubules displayed patchy degenerative and regenerative changes including loss of apical brush border, nuclear enlargement and prominent nucleoli, and few interstitial foam cells (Figure 2). By immunofluorescence microscopy, glomeruli stained for IgG, C3, C1 and kappa light chain. There was no staining for lambda light chain. Stains for IgG heavy chain subtypes were positive for IgG1 only (Figure 3), consistent with monoclonal IgG1-kappa deposition. Electron microscopy revealed electron dense deposits in the mesangium, also in subendothelial and subepithelial locations in the capillary walls. On high-power magnification, these deposits revealed a microtubular substructure (i.e., with a central lumen) (Figure 4). These pathologic findings were consistent with ITG.
Findings of ITG with IgG1 deposit as well as bicytopenia with relative lymphocytosis warranted further workup for gammopathy. SPEP revealed nonselective protein loss (total protein was 4.5g/dL, Albumin 2.9g/dL, total globulin 1.6g/dL, Albumin/Globulin ratio 1.8) with non-detectable M spike. UPEP was negative. IgG was 203mg/dL, IgA 45mg/dL, IgM 50mg/dL. Serum free kappa light chain was 35.79mg/L with normal Lambda light chain level of 20.08 mg/L with Kappa/Lambda ratio 1.78. Beta-2 microglobulin level was 6.4mg/L. Peripheral blood flow cytometry was ordered, which revealed 20% monoclonal B-cells (CD5+/CD23+) with CLL phenotype.
Pt was started on prednisone 60mg daily for 7 days for ITG and decision was made to start CLL-targeted treatment with Obinutuzumab for 6 cycles, with the intent of improving the glomerulopathy, as pt did not meet IWCLL-2008 criteria for initiation of treatment for CLL. Obinutuzumab was given in the usual schedule of 100 mg on day 1, 900 mg on day 2 and 1000 mg on day 8 and 15 of first month, followed by 1000 mg every 28 days for 5 additional cycles. Repeat flow cytometry in 6 months after diagnosis showed complete response of monoclonal lymphocytosis (minimal residual disease negative). There was complete normalization of kidney function (Cr 0.73mg/dl and glomerular filtration rate 78ml/min) and no further evidence of proteinuria or edema. Hypertension has fully resolved, without additional need for anti-hypertensives. 30 months post treatment pt remains in remission for both MBL and nephrotic syndrome.
Discussion:
Treatment with obinutuzumab may lead to complete resolution and sustained remission of nephrotic syndrome associated with CLL. The more efficient B-cell depletion achieved with obinutuzumab may make this a better treatment choice for CLL associated NS than rituximab. Further evaluation with clinical trials is warranted.
Skarbnik: Novartis: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Genentech: Speakers Bureau; Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.